Solid Sodium Chloride Dissolves in Water
It comes as a dissolved solid associated with sodium and calcium as carbonates or. A familiar example of an ionic compound is table salt or sodium chloride.

Water Molecules And Their Interaction With Salt U S Geological Survey
Which statement is true.

. For example the purity of the pharmaceutical grade must be higher than. You begin pouring sodium chloride into a glass of water. Impurities of sodium carbonate may include water 15 sodium chloride 05 sulphate 01 calcium 01 magnesium 01 and iron 0004.
In solid ionic compounds the ions are too tightly held by chemical bonds and cant flow from their ordered situation. In his answer to this question the late John Margrave argued that salt dissolves in water as ions of sodium and chlorine and. Aqueous silver nitrate solution AgNO 3aq contains.
Based on the StoPGoPS approach to problem solving What is the question asking you to do. Salt has a high melting point of 800ºC. While a salt crystal is an electric insulator saline solutions salt dissolved in water readily conduct electricity.
Sodium chloride octahedral 66 The sodium chloride NaCl polymorph is most common. Corrosive when it dissolves in condensate. It appears as a solid clear crystal with little or no odor.
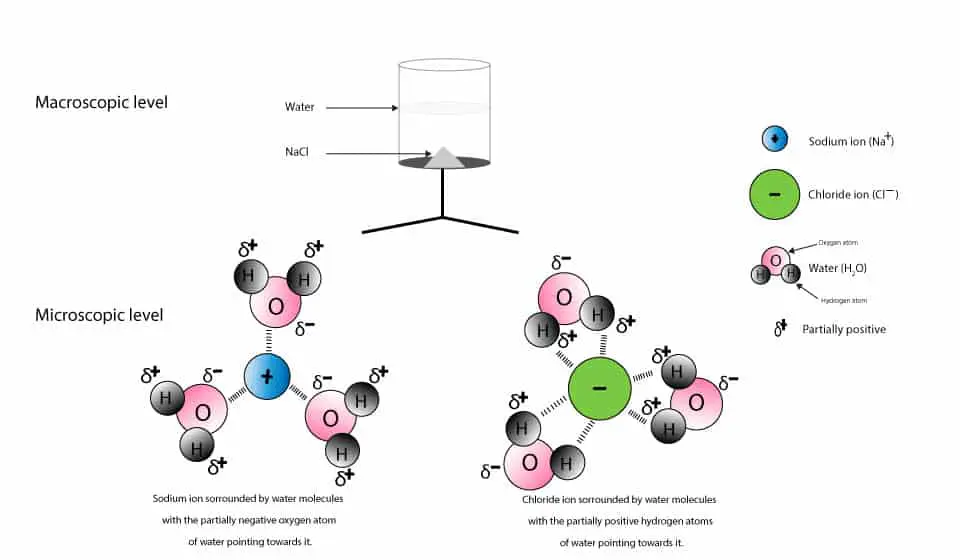
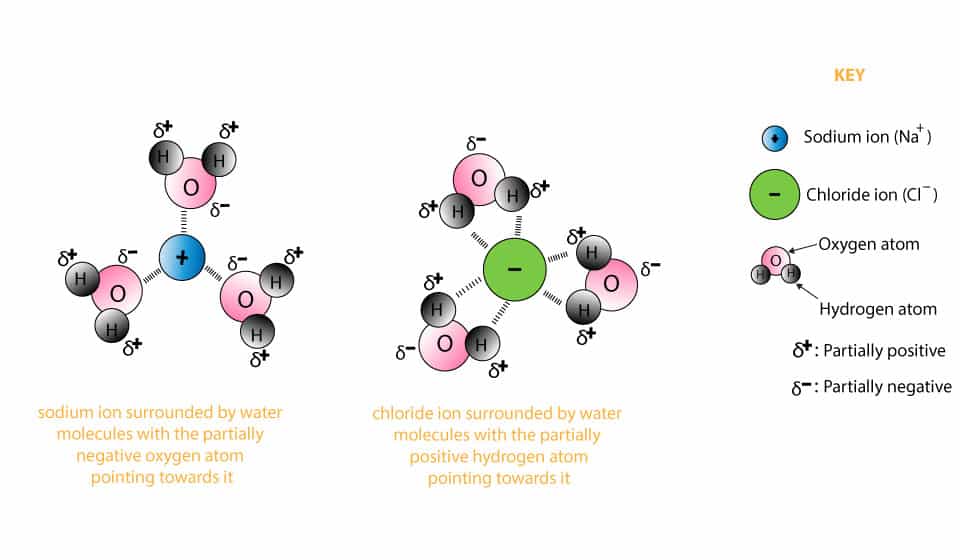
A cubic close-packed arrangement of chloride anions with rubidium cations filling the octahedral holes describes this polymorph. Each NaCl molecule is surrounded by water molecules. When an ionically bonded substances are melted or dissolved in water the ions are free to move about.
The purity and the impurity profile depends on the composition of the raw materials the production process and the intended use of the product. Sodium chloride has a molar mass of 5844 grams per mole. Sodium chloride completely dissociates in water to form sodium ions and chloride ions.
The given set up of the figure is for preparation of an acid. In even more simple terms a simple ionic compound with positive and negative ions such as sodium chloride common salt is easily soluble in a highly polar solvent with some separation of positive δ and negative δ- charges in the covalent molecule such as water as thus the sea is salty as it accumulates dissolved salts. Determine whether sodium chloride is a strong electrolyte or a weak.
As a salt sodium chloride dissolves well in. Hydrogen chloride HCl dissolves in water to form ionic hydrochloric acid HCl-aq 2 The. It is an ionic solid made up of sodium ions.
C Why empty flask is used. Depending on conditions solid RbCl exists in one of three arrangements or polymorphs as determined with holographic imaging. In presence of a drop of water HCl gas dissolves in water and forms hydrochloric acid which turns blue litmus paper red.
Each sodium ion is surrounded by chloride ions. Pitting is often evident in kiln piping where condensation occurs in systems where carbon dioxide is a problem. Acute exposure to sodium cyanide may require decontamination and life support for the victims.
A Name the acid prepared by this method. Is sodium chloride a strong electrolyte or weak electrolyte. However some covalent substances dissolve in water and form ions.
For a long time the sodium chloride just dissolves in the water but suddenly it begins to pile up at the bottom of the glass. Aqueous sodium chloride solution NaCl aq contains sodium ions Na aq and chloride ions Cl-aq. B Name the reactants used.
F Hydrogen chloride is not collected over water as it is highly soluble in water. Molten salt is also a conductor. Lacrimation tearing and a burning sensation of the mouth and throat are common.
Sodium cyanide is irritating to the skin and mucous membranes. D What is. Increased salivation nausea and vomiting are often seen.
Keep in mind that the source of most carbon dioxide is not a dissolved gas in makeup water removable by deaeration. Dissolution of sodium chloride in water. If a salt is soluble in water it dissolves in water by breaking up into its ions which are completely surrounded by water so the species in water are not salt molecules but cations and anions surrounded by water molecules.
If you examine salt crystals with a magnifying glass you can observe the regular cubic structure resulting from the.
How Does Sodium Chloride Nacl Dissolve In Water

0 Response to "Solid Sodium Chloride Dissolves in Water"
Post a Comment