In an Oxidation Reduction Reaction the Substance Reduced Always
Reduction is the GAIN of ELECTRONS by atoms or ions of a substance 2. 58 A gains electrons B becomes a charged species C takes on oxygen atoms D gives up hydrogen atoms E loses electrons.

Oxidation And Reduction Reactions Basic Introduction Youtube
In an oxidation-reduction reaction the substance that is reduced always shows a loss of electrons.

. Using this idea say which substance is oxidised and which substance is reduced in each reactioni sMg gO2. When oxygen typically is reacted with a hydrocarbon fuel the oxygen is formally reduced to C O 2 in which the oxygen number of O is I I REDUCED from the zerovalent elemental state. The OXIDANT is always reduced in a redox reaction.
Reaction magnesium is oxidized and chlorine is reduced. Because electrons are neither created nor destroyed in a chemical reaction oxidation and reduction always occur in pairs it is impossible to have one without the other. 24 In this reaction what is the substance oxidized.
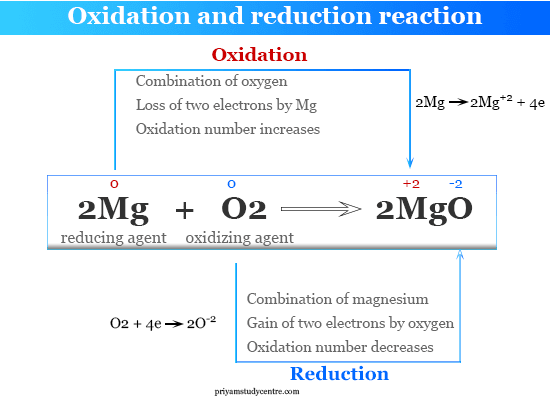
2Mg s O 2 g 2MgO s is a Oxidation reaction. 2O2-aq - O2 g 4e-Oxidation. The element that ACQUIRES electrons BECOME MORE NEGATIVELY CHARGED 3.
Which of the following concerning oxidation-reduction reactions isare correct. In an oxidation-reduction reaction bartleby. Shows a gain of electrons.
In the below reaction Magnesium gets oxidized by losing two electrons to oxygen which gets reduced by accepting two electrons from magnesium. Oxidation number DECREASES and is said to be reduced. Shows a gain of electrons.
MgO s C s Mg s CO g is a Reduction reaction. In the following half reaction what class of reaction is shown. Up to 256 cash back In an oxidation-reduction reaction the substance reduced always.
In the Mg Cl 2 reaction Cl 2g is the oxidizing agent because it is reduced and in the process it oxidizes removes electrons from the magnesium metal. It itself is oxidized. It itself is reduced.
Takes on oxygen atoms. In an oxidation-reduction reaction the substance reduced always a takes on oxygen atoms. A chemical substance that gives up oxygen or takes on electrons from another substance.
C gives up hydrogen atoms. D shows a gain of electrons. The reactant in a redox reaction that causes another substance to be reduced.
O takes on oxygen atoms. 58 In an oxidation - reduction reaction the substance oxidized always ________. Magnesium undergoes both oxidation and reduction processes.
Oxidation-reduction reactions always form gaseous products. At least one substance is oxidized and one substance is reduced in an oxidation-reduction reaction. Obecomes a charged species.
In an oxidation-reduction reaction the substance reduced always takes on oxygen atoms. The substance that gains electrons in the reaction ie. Becomes a charged species.
O gives up hydrogen atoms. Shows a gain of electrons. Gives up hydrogen atoms.
Becomes a charged species. The OXIDANT is always reduced a redox reaction. Chemistry questions and answers.
Science Chemistry Chemistry questions and answers In an oxidation-reduction reaction the substance that is reduced always takes on oxygen atoms. O shows a loss of electrons. Chemistry questions and answers.
QUESTION 18 In an oxidation-reduction reaction the substance that is reduced always takes on oxygen atoms. B shows a loss of electrons. A compound of H-1 with group I.
The reactant in a redox reaction that causes another substance to be oxidized. Add the appropriate elements given above depending on the type of solution to balance. A process in which a chemical substance takes on oxygen or loses electrons.
Chemistry questions and answers. Gives up hydrogen atoms. Obecomes a charged species.
Gives up hydrogen atoms. The species that is oxidized loses one or more electrons in an oxidation-reduction reaction. Becomes a charged species.
What is the oxidation number of a free element. Reduction reaction is defined as anything that leads back to a free metal state. 23 In an oxidation-reduction reaction the substance oxidized always B shows a loss of electrons.
Formally a species that gains electrons is reduced and a species that loses electrons is oxidized. Takes on oxygen atoms. O shows a loss of electrons O shows a gain of electrons.
Oxidation and Reduction always take place together so that if one substance is oxidized another is reduced. 59 In an oxidation - reduction reaction the substance reduced always ________. When oxygen typically is reacted with a hydrocarbon fuel the oxygen is formally reduced to CO2 in which the oxygen number of O is I I REDUCED from the zerovalent elemental state.
E becomes a charged species. Click hereto get an answer to your question If a substance gains oxygen during a reaction it is being oxidized. If it loses oxygen it is being reduced.
Is reduced can also be called the oxidizing agent. 59 A loses electrons B gives up hydrogen atoms C gains. Question 1 In an oxidation-reduction reaction the substance reduced always O gives up hydrogen atoms.
Oxidation means a gain of oxygen and Reduction means loss of oxygen. An oxidation-reduction reaction in which a metal is oxidized and oxygen is reduced usually in the presence of moisture. In an oxidation-reduction reaction the substance oxidized always.
Shows a gain of electrons. O gives up hydrogen atoms. Oxidation is the loss of electrons.
In an oxidation-reduction reaction the substance reduced always. Almost always 1 In hydrides -1. Zn s 2 HCl aq ZnCl2 aq H2 g D zinc 25 In an oxidation-reduction reaction the substance reduced always D shows a gain of electrons.
Shows a loss of electrons. O becomes a charged species. Formally a species that gains electrons is reduced and a species that loses electrons is oxidized.
Shows a loss of electrons.
Oxidation Reduction Reaction Definition Concept Examples

Dissolved Molecular Hydrogen Or H2 In Kangen Water Is An Antioxidant Scavenging For And Neutralizing Hydr Kangen Water Benefits Kangen Water Water For Health
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
0 Response to "In an Oxidation Reduction Reaction the Substance Reduced Always"
Post a Comment